Neumifil


About Neumifil
Designed to inhibit viral adhesion to the epithelium of the nose, preventing virus-induced exacerbations of underlying respiratory diseases.
Neumifil is a self-administered, easy to use, intranasal spray developed from our proprietary GlycoTarge™ platform. It is locally acting to inhibit viral infection through the nose, blocking and preventing viruses from gaining entry and infecting the host, thus preventing virus induced exacerbations.
Neumifil is a novel, engineered multivalent Carbohydrate Binding Module (mCBM40), which is being developed to offer at-risk patients, suffering from chronic pulmonary diseases, protection from viral induced exacerbations of their underlying respiratory diseases. With its unique mechanism of action, Neumifil potentially offers reduced susceptibility of the virus developing resistance to the medication, such as that observed to develop against directly acting anti-virals or vaccines.
Neumifil science
Neumifil, is a multivalent, sialic acid-binding protein engineered from the Carbohydrate-Binding Module (CBM) of streptococcus pneumoniae neuraminidase A protein (SpCBM). Multivalency with respect to sialic acid-binding is achieved by fusing two tandemly linked SpCBM domains to an oligomerization domain, derived from Pseudomonas aeruginosa sialidase, that self-associates to form a trimer.
By directly targeting sialic acids on host cells in the nose, Neumifil blocks viral entry to cells and the establishment of infection.
Given this novel host-targeted mechanism of action, Neumifil has reduced the potential for the development of drug resistance developing in the viruses.
For viruses bearing surface glycoproteins including SARS-CoV-2, Neumifil has a second mechanism of action that can prevent viral entry. In addition to blocking the sialic acid containing cellular receptor, Neumifil can also bind to viral protein glycans with high affinity, preventing entry to the host cell.
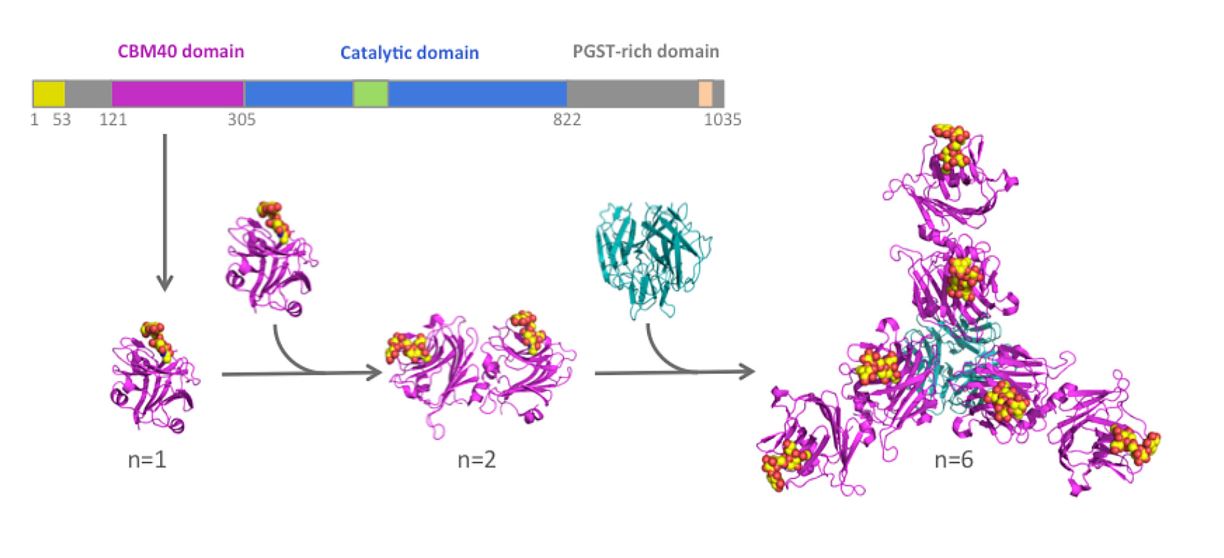
A gene coding for a single sialic acid binding module is derived from a gene encoding a Streptococcus pneumoniae neuraminidase (NanA). Two of these genes are tandemly-linked using DNA coding for a short connecting peptide. This gene is then linked to a gene that codes for a small protein domain which forms a trimer in a Psuedomonas aeuriginosa sialidase. When expressed in E. coli, the trimerisation domains self-associate creating a protein with six sialic acid binding domains. The affinity for sialic acid increases several thousand-fold when going from the monomer to the hexamer.
Programmes
Preclinical data
In multiple animal studies, Neumifil demonstrated an excellent safety profile and broad efficacy against a wide range of respiratory viral pathogens, including Influenza Virus, Respiratory Syncytial Virus, and Coronavirus.
Neumifil has demonstrated high-affinity binding to cellular sialic acids and/or virus proteins and can effectively prevent cell entry and infection in in-vitro assays. When administered prophylactically or therapeutically in-vivo, Neumifil has significantly improved animal clinical outcomes and demonstrated protection against virus-challenge in multiple animal models.
Clinical data
Positive top line results from Phase 1 data
Pneumagen undertook a first-in-human, placebo (vehicle) controlled, single ascending dose; multiple ascending dose study of Neumifil in healthy volunteers. A total of 60 participants were recruited. Single dose and multiple doses (daily dosing for seven days) were administered to the study participants. The study was designed to assess the safety and tolerability of Neumifil. Safety was assessed between each dose cohort.
Systemic exposure of Neumifil and effect of Neumifil on cytokine concentrations were also assessed.
The study demonstrated:
- Single and repeated intranasal doses of Neumifil were well-tolerated in healthy volunteers.
- There were no clinically significant findings or changes in clinical laboratory variables, the results of nasal and physical examinations, vital signs, or ECGs, during the study.
- Neumifil was not detected in any of the blood samples collected, indicating systemic exposure does not occur or the concentrations are undetectable.
- Data from the study supported continued development.
More information can be found at https://www.clinicaltrials.gov/ct2/show/NCT05093530?term=pneumagen&draw=2&rank=2
Statistically significant Phase 2 results
Pneumagen evaluated Neumifil in a Phase 2 Controlled Human Infection Model (CHIM) where participants were challenged with influenza virus following administration of Neumifil.
Neumifil demonstrated a clinically and statistically significant reduction in both the incidence of symptomatic influenza infection and in the severity of symptoms compared to placebo. Neumifil was well tolerated with no new emergent adverse events or safety signals from the study compared to the first in human study.
Based on these positive clinical results, alongside the pre-clinical data package demonstrating Neumifil’s activity against a broad range of viruses, Pneumagen plans to advance Neumifil into further clinical studies. These will include the evaluation of Neumifil’s ability to reduce the incidence of viral induced exacerbations in patients with Chronic Obstructive Pulmonary Disease (COPD) where they represent a significant unmet clinical need.
Read full press release here: Pneumagen Announces Successful Clinical Proof of Concept for its Broad-Spectrum Antiviral Neumifil in Phase 2 Influenza Human Challenge Study – Pneumagen
Market opportunity
There is a significant medical need for the prevention and treatment of virally induced exacerbations in patients with underlying respiratory diseases such as COPD, as well as for patients who have weakened immune systems including the elderly and immunocompromised.
The exacerbation of disease caused by respiratory viral infections can accelerate lung function decline and lead to greater morbidity and mortality rates as well as significantly higher healthcare costs.
Neumifil is being developed to prevent viral respiratory infections in patients with chronic respiratory diseases, and thereby potentially reducing the disease burden for patients who currently have limited prevention options.
The shortcomings of current treatments
Corticosteroids (inhaled and oral) are commonly prescribed in the management of asthma and COPD patients by reducing inflammation. However, common side effects of oral steroids include, weight gain, indigestion, insomnia, feeling restless, sweating a lot and mild mood changes. Serious long-term effects include, diabetes, adrenal gland suppression, hypertension and glaucoma. Inhaled steroids can result in oral thrush and hoarseness.
For those patients that have frequent exacerbations, prophylactic antibiotics are administered to help reduce the frequency of exacerbations and admissions to hospital. However, side effects and the potential for the development of antibiotic resistance is a serious public health problem and is a considerable cost to payors.
Overall, there are no current drugs on the market that address the stopping of the virus in the nasal passages, with the potential to result in a significant reduction of exacerbations.
Neumifil addresses a significant unmet need
The National Institute of Clinical Excellence estimate 1 in 8 emergency admissions to hospital in the UK are for COPD (130,000 per year). Many more experience exacerbations that do not require hospitalisation. The US COPD foundation estimate 30 million Americans are affected by COPD. A paper reported 1.2 million hospitalisations in the USA in 2006. An exacerbation means further exacerbations are more likely leading to increased morbidity and health care utilisation.
Exacerbations not only affect the quality of life at the time of exacerbation they contribute to declining lung function.
Partnership with COPD Foundation
The Foundation’s mission is to prevent COPD, bronchiectasis, and nontuberculous mycobacterial (NTM) lung disease, and to seek cures while improving lives and advocating for all affected.Pneumagen supports the Foundation as a member of the 2022 Corporate Partners Program.
To learn more, please visit the Foundation at www.copdfoundation.org






 Website powered by 100% renewable energy
Website powered by 100% renewable energy